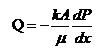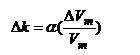Factors – Coal
Factors 0 CommentThe strength and elastic module of the coal seam influence the fracture mechanics of an outburst. Stored elastic strain energy is released, and the failure is usually brittle indicating that the strain energy is not being used to distort the strata and a large proportion is still stored at the moment of breakage. Therefore, the ability of the seam to accumulate elastic strain energy is a large factor in assessing the liability of an outburst in the seam. (Lama & Bodziony, 1996)
A commonly used index for the ‘bursting liability’ of a coal seam is the strain energy index,known as “Wet”. Wet is the ratio of accumulated elastic strain energy to the permanent strain energy. The Wet values for a particular seam may be found either through laboratory experiments or by in-situ evaluations, with a Wet value greater than 5 indicating a seam is severely prone to outburst. The strain energy for an entire seam can be found by taking the mean of the values obtained for samples in key areas of the strata.
Rank of coal seamCoalification or metamorphosis of coal is defined as gradual changes in the physical and chemical properties of coal in response to temperature and time. The coal changes from peat through lignite and bituminous coal to anthracite. With extreme metamorphisom, anthracite can change to graphite. The rank of coal is the stage the coal has reached on the coalification path. The changes, with increasing rank, include an increase in carbon content, and decreases in moisture content and volatile matter as shown in the table below. |
COAL RANK |
CARBON CONTENT ( %) |
VOLATILE MATTER ( %) |
CALORIFIC VALUE (kJ/kG) |
MOISTURE CONTENT% |
|
PEAT
|
60
|
>53
|
16800
|
>75
|
|
BROWN COAL
|
60 – 71
|
53 – 49
|
23000
|
35
|
|
SUBBITUMINOUS COAL
|
71 – 77
|
49 – 42
|
29300
|
25 – 10
|
|
BITUMINOUS COAL
|
77 – 87
|
42 – 29
|
36250
|
8
|
|
ANTHRACITE
|
> 87
|
29 -8
|
>36250
|
<8
|
|
–
|
On dry ash free basis
|
On dry ash free basis
|
Ash Free Basis
|
In-situ
|
Coalification of coal is generally a consequence of thermal effects and compaction.The coalification processes involved in coal formation are marked by a well defined progression of increasing rank. Coal rank increases with depth, and the combination of depth of burial and geothermal gradient essentially determine the rank of coal. Water, carbon dioxide and methane are generated during the progressive coalifciation. Water and carbon dioxide are produced during coalification of lower ranks of coal.
Methane is the predominant gas generated in the bituminous coal and anthracite stages of coalification, and the carbon dioxide produced at lower ranks is typically flushed out of the coal by methane. The sorption capacity of coal increases with rank. Usually high rank coal can absorb more gas and the adsorptive capacity of coal for methane increases with coal rank . The sorption capacity of coal can be influenced by different intrusions and by the tectonic events such as folding and faulting. Coals near igneous intrusions such as dykes, may contain calcites and pyrites which are likely to influence the drainability of gases.

Permeability is a physical property of porous materials, which determines the flow of fluid through the material by an applied pressure gradient. It may be described as the “fluid conductivity” of the porous material. Permeability is also used to describe the resistance of strata to the passage of gas through it (Mordecai and Morris, 1974).
One of the principal physical parameters governing gas emission from coal is cleat permeability (Jones et al., 1982). To properly plan the gas drainage system in the mine, information about gas pressure, gas composition, location of the gas reservoirs and permeability of the seam is required. Highly permeable coal offers good opportunity to recover methane. The unit of measuring the permeability is the Darcy. The Darcy unit represents the flow capacity required for 1 ml of fluid to flow through 1cm2 for a distance of 1 cm when 1 atmosphere of pressure is applied. The permeability values vary according to gas type and differential pressure across the coal seam
Permeability of coal can be calculated using the following Darcy’s equation:
where:
k = Permeability, Darcy
dp/dx= Pressure gradient, atm/cm
A = Cross sectional area, cm2
= Viscosity of the fluid, centipoise
Q = Volumetric flow, cm3/s
Australian coals are commonly of low permeability (less than 5 md) compared with coal seams mined in other countries,and represents a significant problem for the exploitation of the gas. Hayes (1982) reported that the Bulli seam permeability is considerably less than 1 mD. Lingard, Phillips and Doig (1982) reported permeability of Australian coals from Appin, West Cliff and Leichhardt (now closed) collieries that varied from less than 0.1 mD to 100 mD.
The following factors influence the coal permeability (Enever and Hennig, 1997)
Effective stress
In situ stress fields and coal permeability are closely interrelated. In fractured reservoirs, such as coal-beds, the permeability of coal is sensitive to stress variation or pore pressure. The permeability of a particular coal was found to depend on the level of the confining stress, for example a permeability of 12 mD at 0.07 MPa confining pressure changed to 0.0035 mD at 20 MPa. Somerton, Soylemezoglu and Dudley (1975) found that increased applied stress in constrained coal samples caused a decrease in permeability of several orders of magnitude, up to the point where microfracturing occurred, and permeability increased again beyond this point.
Limited tests on measuring the permeability of coal have been undertaken on different Australian coals. The measured permeability for the Bulli coal seam under triaxial conditions varies from 0.001 mD (Lingard, Phillips and Doig, 1984) to 0.15 mD (Somers, 1993). Their results also showed that in all tests the permeability decreased with increasing stress. Xue and Thomas (1991) investigated the variation of Australian coal sample permeability with confining stress and changing gas mean pressure. They showed that the permeability of coal increased when the mean gas pressure was decreased. However, the coal samples were one to two orders of magnitude smaller in permeability as the confining stress increased from 1 MPa to 15 MPa.
Somers’s (1993) work on Australian coal permeability found that under 5 MPa confining pressure, Bulli coal samples had an average permeability of 30 mD, with Westcliff coal 21mD, Tower 45 mD, and Tahmoor 17 mD. Ulan coal permeability is around 16 mD. Gray (1995) (ACARP Report C 3079, page 331) also presented a relationship between effective stress and permeability for cores taken from Leichhardt Colliery in the Bowen Basin.
Coal petrography
The permeability of coal seams can be influenced by geological structure variations. Coal seam permeability is affected by the changes in a geological structure of coal particularly in the vicinity of vault, dyke or fold. The level of coal permeability is influenced by the severity of the structural variations. Generally, favourable areas for coal bed methane drainage are likely to have a relatively simple geological structure to ensure the continuity of reservoirs. Gently folded areas in coal seams tend to have higher permeability than steeply folded and faulted areas. Cleat system in gently folded coal is likely to have the cleats open and continuous, thus facilitating increased permeability. However, in the structurally complex areas of dykes and faults, the cleat system tend to be severely damaged leading to low permeability,especially where severe structural compression has occurred.
The permeability among different coal litho-types varies even under the condition of similar coal rank. The coal petrological composition affects the overall permeability of the coal-bed through controlling the development of the pore and fissure system. The permeability of a vitrinite-rich coal reservoir is around 10 times higher than an inertinite rich one (Symth, 1993). Also, the inertinite-rich coal absorbed more methane than a middle-rank coal sample, but absorbs the same amount as a high rank coal sample.
Bartosiewicz and Hargraves (1984) examined various coal samples from Australian coal basins, and the results showed significant variations in permeability in different directions. Bedding plane permeability is significantly greater than the permeability normal to the bedding. However, Lingard, Phillips and Doig (1982) reported no significant difference between coal samples cut parallel and perpendicular to the bedding plane. Gash et al. (1993) tested the permeability of American coal samples and found that the permeability in the face cleat direction was greater.
Mineralisation
There are some differences between low permeability and high permeability coal seams. These differences can be related to the presence of some specific pore and cleat fillings such as mylonite, the development of cleats and their mineralisation, and the mode of occurrence of minerals in coal macerals. Successful drainage and a suitable rate of gas flow through the coal can be influenced by coal microstructures, especially micro-cleat openings and mineral matter. In good drainage and high permeability coal seams, the micro-cleats are mostly empty, or only partly mineralised.
In coal seams with negligible quantities of mineral matter, the coal-bed gases flow and initially continue to flow when the pressure is lowered below the desorption pressure. The coal matrix shrinkage as a result of gas desorption may cause greater cleat openings than the effective stress. Titheridge (2004) stated, “In mineralised coal, the presence of calcite (or other minerals) in cleat or fractures adds an additional factor to the initial and subsequent drainage process. Mineralisation blocks cleat and fracture permeability routes that would otherwise transport gas”. Thus, an increased presence of mineral matter in coal would cause a reduction in coal permeability, and the degree of permeability will be proportional to the extent of mineralisation. Furthermore, mineral matter impedes the gases from leaving their place by affecting the desorption and shrinkage properties of the coal matrix.
Gamson, Beamish and Johnson (1993) study on Australian coals found that the amount of fracture infilling with minerals was one of the factors which influenced the effectiveness of methane flow through the coal matrix. They also noted that mineral matter such as clay, calcite and quartz block the methane flow path through cleats and interconnected pores by forming a compact amorphous or crystalline structure. The size of infillings influences gas diffusion as well as laminar flow in the coal matrix. Later Gurba (2002b) described her microscopic studies of some Australian coal samples and found that the Bulli seam had two different sets of cleats. One set of cleats is open and the other mineralised. Microscopic studies on coal samples from West Cliff Colliery showed micro-cleats totally mineralised by carbonates. Siderite nodules (Iron Carbonate) in the cleats were observed to cause difficulty in drilling and in drainage. Mylonite is also present in West Cliff coal samples, and mylonitic type coal could be prone to outbursts (Gurba, 2002a). Additionally, microscopic examination of the coal samples from difficult drainage areas has shown that the presence of mylonite in micro-cleats is also likely to cause difficulties in gas drainage. As revealed by electron microprobe analysis the mylonite in micro-cleats is cemented by calcite, dolomite or kaolinite. In the coal samples from Central Colliery in the Bowen Basin that were collected from the low permeability area and outburst prone zone, the cleats were totally filled with calcite. In Appin Colliery coals, carbonates were present in the cleats as well as mylonite, which was cemented by carbonates so that there was not much space for gas flow. Titheridge (2004), who did extensive work on Tahmoor Colliery Bulli coal and its calcite mineral matter, postulated that high fluid pressure was the major factor responsible for the fibrous veins in coal (sedimentary rock). He stated, “The origin of high fluid pressure was primarily due to the fluctuating NE-SW tensional – compressive stress field that was present during the burial phase of the Southern Sydney Basin”. Calcite in Tahmoor coal was formed from the combination of CO2 and water, for which one of the CO2 resources was magmatic.
Degree of fracturing
Coal seams have natural fractures, known as cleats. Cleats act as a major transport system for gas and water flow within a coal seam. There are two sets of cleats in coal, face and butt cleats. Face cleats are longer than butt cleats, hence directional anisotropy in coal permeability results from this phenomenon. Permeability of coal increases with cleat density and cleat width. The flow capacity of fractured media depends almost entirely on the number and width of fractures and their continuity (Dabbous et al. 1974). Lingard, Phillips and Doig (1984) showed that the dimensions of fractures influence coal permeability. The greater the fracture, the higher is the permeability of the coal. Flow through cleats is generally laminar flow.
Information relative to the cleat size and spacing in coal are useful in predicting permeability,and generally the larger the cleat size and cleat density, the higher the permeability. Secondary cleats also occur in coal as a result of induced stress and changes to coal geological structure or mining. These fractures normally cause permeability to increase, but sometimes they do the opposite and reduce the permeability, and such situations tend to occur in shear zones or near magmatic intrusions. According to Hayes (1982) permeability in the fractured and crushed zone ahead of the face side during mining is greater than permeability in the intact and solid coal area.
Gas type and pressure
In the case of methane and carbon dioxide, increasing gas pressure decreases the permeability, but this reduction is greater for methane than carbon dioxide. This effect is remarkable at the lower pressures for both gases, but by increasing the pressure up to 7.5 MPa for carbon dioxide, the rate of decrease drops further. As for methane, the permeability decreases to half of its initial amount when the gas pressure increases from near 0.5 MPa to 2 MPa.
The ratio of CH4/CO2 in Australian coal seams varies; in some deposits CO2 is the predominant gas, and in other areas methane is predominant. The permeability of coal is higher for methane than carbon dioxide (Lama, 1995a; Bartosiewicz and Hargraves, 1985). Australian researchers Xue and Thomas (1995) investigated the permeability of Australian coals to a mixture of CH4/CO2. They stated that by using the Darcy and Dalton pressure laws, the permeability of coal to a mixture of two gases can be derived theoretically from the following Equation:
N1 = Volumetric percentage of gas No 1
N2= Volumetric percentage of gas No 2
K1= Permeability to gas No 1, Darcy
K2= Permeability to gas No 2, Darcy
Km= Permeability of coal to mixture, Darcy
= Viscosity of gas No. 1, centipoise
= Viscosity of gas No. 2, centipoise
= Viscosity of mixture gas, centipoise
Xue and Thomas (1995) found the difference between the theoretical calculated and the experimentally measured permeability was about +/-15%. They also reported that by increasing the proportion of carbon dioxide in the mixture, the permeability of coal decreases till the point which the composition of mixture was approximately 60% CO2 and 40% CH4 and then begun to increase.Finally they explained the adsorption effect on the permeability of coal to methane and the carbon dioxide mixture by defining the mutual and individual adsorption effects, which are the effects of adsorption of a gas from a mixture and from an individual gas on coal permeability, respectively.
Water
In virgin coal seams, water normally fills pore spaces, cleats, and fractures and any gas present is dissolved within the seam water or absorbed on the internal surface of the coal, while the reservoir and its fluid components are in equilibrium (Van der Meer, 2004). Permeability of a coal seam to gas is dependent on the concentrations of water (Thakur and Davis, 1977). Usually free gas only comes out of the coal into the cleat space when the water pressure drops below the sorption pressure (Gray, 2000). The permeability of coal to water is increased by decreasing the pressure (Dabbous et al., 1974). Kissell and Edwards (1975) reported that the relative permeability of a coal seam increases as the water in the seam decreases, thus making more space available for the gas phase to flow. It means that initially permeability will decrease with a drop in reservoir pressure around the production hole, followed by an increase, as significant desorption induced shrinkage occurs as water and gas are produced from the seam, with the effective stress increases leading generally to a reduction in permeability. However many coal seams exhibit an increase in permeability with production, because of seam de-stressing and coal shrinkage due to gas desorption. Coal shrinkage reduces the lateral stress in the seam and shifts the stress into the surrounding rocks. The opposing effects on effective stress mean that the permeability of the seam may either increase or decrease with the removal of gas and water from the seam.
Generally, permeability is reduced by an increase in moisture content (Bartosiewicz and Hargraves, 1985). However, it should be pointed out that the gas permeability of a coal mass is influenced by the degree to which the permeable volume of the pore is filled with natural moisture. Natural moisture decreases the permeable volume of pores by a factor of greater than two (Ayruni, 1981).
Coal swells and shrinks with gas sorption (adsorption and desorption), the extent of the changes in coal is influenced by gas type and pressure. Volumetric changes in coal are attributed to both coal matrix change and the changes in cleats, fissures and other fractures in coal. These changes are dependent upon the coal rank, maceral structure of coal, the degree of mineralisation, geological disturbances and intrusions.
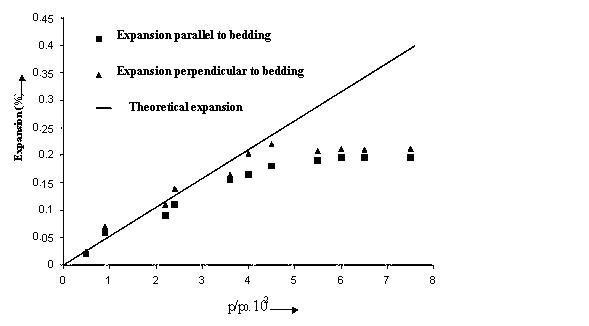
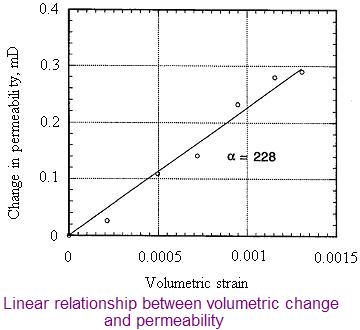
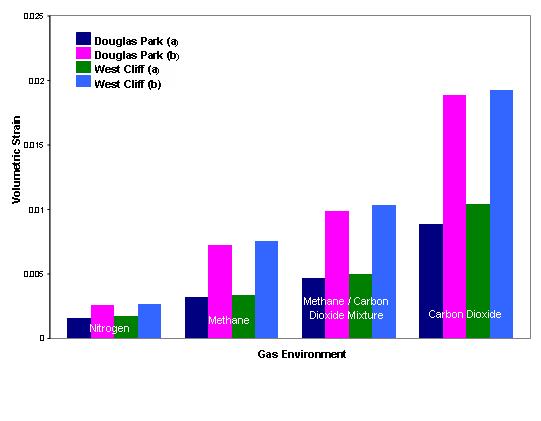
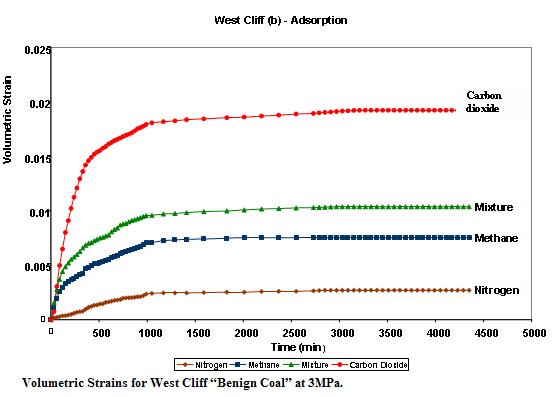
Volumetric changes in coal have a profound effect on coal permeability and porosity.The figure opposite shows the relative volumetric changes in Bulli seam coal from West Cliff colliery ,N.S.W, Australia. The coal samples were tested for different gases under different gas pressures up to 3 MPa.
The tests are usually carried out in a pressure vessels under controlled conditions as shown in the figure below.
The appropriate way of expressing volumetric changes in coal is by shrinkage coefficient, which is defined as the rate of change of the coal matrix volume to the change in gas pressure and is given by:
Vm = Matrix volume, m3
dVm = change in volume, m3
dP = change in applied pressure, MPa
Cm = shrinkage coefficient, MPa-1
Carbon dioxide appears to have the greatest influence on the matrix and nitrogen the least. This is understandable in view of the fact that carbon dioxide has a greater affinity to coal than the other gases.Nitrogen as a neutral gas does not have much effect on the coal volume.
Interest in coal shrinkage and swelling dates back almost 100 years, table below lists various studies undertaken from the turn of the last century to the present day.
Reference |
Gas |
swelling/shrinkage coefficient,MPa-1 |
| Moffat & Weale (1955) | CH4 | 1.17 x 10-8 |
| Gunther (1968) | CH4 | 1.90 x 10-8 – 4.76 x 10-8 |
| Wubben, Seewald and Jurgen (1986) | CH4 | 9.65 x 10-9 – 4.76 x 10-8 |
| Reucroft & Patel (1986) | CO2 | 4.52 x 10-7 |
| Gray (1987) | CH4 | 1.25 x 10-4 |
| Gray (1987) | CO2 | 1.82 x 10-3 |
| Juntgen (1987) | CH4 | 1.77 x 10-6 |
| Harpalani & Schraufnagel (1990) | CH4 | 4.27 x 10-8 |
| Harpalani & Chen (1997) | CH4 | 2.30 x 10-8 |
| St. George & Barakat (2001) | CH4 | 1.20 x 10-3 |
| St. George & Barakat (2001) | CO2 | 5.20 x 10-3 |
| Seidle & Huitt (1995) | CH4 | 1.96 x10-4 |
| Seidle & Huitt (1995) | CO2 | 1.78 x10-4 |
| Levine (1996) | CH4 | 1.30 x10-3 |
| Levine (1996) | CO2 | 5.50 x 10-3 |
| Dunn & Alehossein (2002) | CH4 | 1.77 x 10-3 – 1.95 x 10-3 |
Volumetric change |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
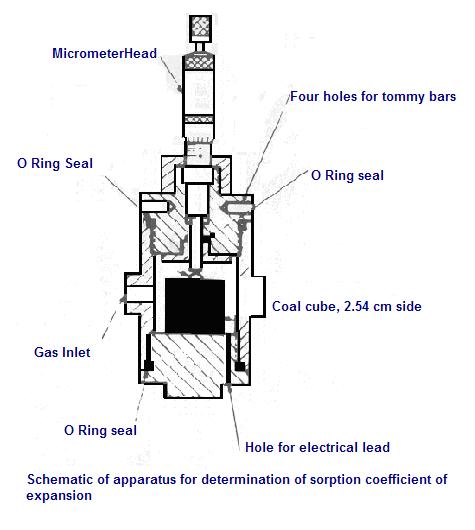
a) Studies prior to 1950In the early 1930’s Briggs and Sinha (1933) tested the swelling and shrinkage of different varieties of UK (Scottish) coals with both firedamp and carbon dioxide, over various pressures ranging from atmospheric to 2.07 MPa (300lbs). Using the specially constructed apparatus shown in opposite Figure.The change in sample size was monitored only along its axis, which was cut parallel with the samples’ bedding. The axial elongation of the coal samples ranged from 0.06% to 0.3% in firedamp and 5% in carbon dioxide gas. Once the gas pressure was removed most samples returned to near original size, however there was an elongation of 0.14% for anthracite. The elongation or shrinkage of the coal was measured by a micrometer, with resolution down to+/- 0.0021 mm. The researchers did not address the radial shrinking or swelling of coal samples in CH4, but recognised that coal can expand in all directions when absorbing gas, reacting less strongly with methane than with carbon dioxide. Coal also absorbs moisture with greater eagerness than methane, and if coal charged with gas is placed in water, much of the gas will be expelled and replaced with water. Others with interest in the field in the pre 1950s period include Meehan (1927), Kvalnes and Gaddy (1931), Audibert (1935) and Coppens (1937). b) Research studies between 1950 and 1960:During the 1950s Moffat and Weale (1955) attempted to define a correlation between the sorption mechanism and the isotherm diagram. They interpreted the sorption mechanism by measuring the coal matrix expansion caused by methane sorption under pressure. Tests were conducted on coal samples parallel and perpendicular to the bedding planes, and were combined to determine the bulk expansion of coal. A constant volume sorption apparatus(opposite figure) was used for the test. Electrical strain gauges, attached to the surface of suitably cut blocks of coal, with connections from the pressure vessels to a Wheatstone bridge were used to monitor coal volume change. The tests were made in methane gas at different pressures ranging between 0 and 70 MPa. Tests were made on different coal types ranging from low rank coal to anthracite. Tests conducted perpendicular to the bedding plane attained a linear expansion ranging from 0.2% to 1.6%, at 15.0 to 20 MPa (150 to 200 atm). Higher rank coal expansion was generally less than for low rank coals. Less expansion was reported on coals tested parallel to the bedding plane. Similar observations were also reported by Audibert (1942) and confirmed by De braaf, Itz and Mass (1952). c) Research between the 1960s and 1970s :Hargraves (1963a) conducted a series of tests on the Bulli seam coal at Metropolitan Colliery, NSW. Using a simplified shrinkage apparatus as shown in the Figure, he was able to determine the sorption coefficient of expansion of the Bulli coal to be approximately 1.75 x 10-3cm/cm at 1 MPa. The test was conducted using 25.4 mm (1 in) cubes subjected to CO2 gas pressures. Gunther (1968) investigated swelling of different ranks of coal using both methane and carbon dioxide, and calculated the swelling coefficients for different types of coal, ranging from low rank, high volatile coals to anthracite. He observed greater swelling of coal in CO2than in CH4. The reported swelling coefficients ranged from 1.90 x10-8 to 4.76 x 10-8 MPa-1 (2.76 to 6.90 E-6 psi-1). Czaplinski (1971) examined the relationship between the kinetics of sorption and the swelling of coal. Tests were conducted parallel and perpendicular to beddings. He showed that at low pressures, the sorption of carbon dioxide was faster than the development of swelling strain, but at higher pressures (> 4 MPa) the two occur simultaneously. Czaplinski’s (1971) studies, stated that “the delay in coal dilation at the initial low pressure levels, causes the gas to enter the coal macro-pores, causing a minimum of volume change. Increased swelling of coal takes place when the gas, at higher pressures, is forced into the micro-pores.” Ettinger (1977) carried out extensive studies on the swelling of different ranks of coal from deposits in Russia, Ukraine and other former Soviet republics. Table below depicts data on coals from the Donetsk basin and other regions of the Ukraine. The stresses due to swelling cause the release of free energy in coal which, according to Ettinger; contributes to the onset of outburst.
d) Research in the 1980’sReucroft and Patel (1983) measured the surface area and porosity of coal with respect to sorption with different gases and vapours, in a similar way to the tests conducted by Mahajan (1982), which measured the porosity as a function of volumetric change. The following equation was used for measuring the volumetric changes of coal:
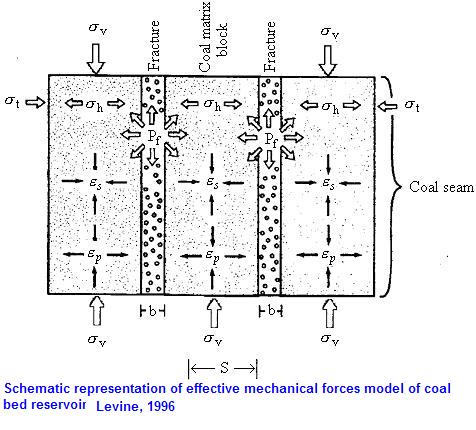
V2 = Volume fraction of coal in the swollen coal sample at equilibrium qw = Mass of coal at equilibrium/initial mass. Q= Swelling parameter (swollen volume/ unswollen volume) Reucroft and Patel (1983) found that the apparent surface area was higher with CO2 in comparison with other gases such as Nitrogen. Using the weighting method, they estimated the volumetric changes of coal, but without mentioning in which direction it happened and by how much. Later, Reucroft and Patel (1986) investigated the swelling of coal, which was induced by gas, in order to obtain a better understanding of the internal structure and surface area of coal. They observed the swelling of various coal samples exposed to different gases such as He, N2 and CO2. The coal samples 1 cm long and 0.4 cm in diameter showed an increase in their length between 0.36% and 1.31% when exposed to carbon dioxide. Insignificant changes were observed when the coal was tested in both nitrogen and helium under the same conditions. Further experiments were conducted under a low pressure of 0.14 MPa, which is rather low compared with the existing in-situ coal gas pressures. Gray (1987) examined the relationship between shrinkage, fluid pressure /effective stress and coal permeability. Gray stated, “shrinkage of the coal occurs on desorption, leading to an effective stress reduction. This opposes the effective stress increase that would normally be expected with a lowering of fluid pressure. Because permeability is a function of the effective stress, it may increase or decrease with stress changes associated with drainage”. This implied that shrinkage of the coal matrix associated with desorption opens up the cleat and results in an increase in the permeability of the coal. Sethuraman (1987) studied the effect of gas pressure on the changing dimensions of coal. He found that there was a linear correlation between swelling and pressure. For methane pressures up to 1.5 MPa, there was a reported volume increase between 0.75% and 4.18%. He also found that the lower the carbon content, the higher the swelling of coal. e) Studies in the 1990sStefanska (1990), a Polish researcher, tested some coal samples using both methane and carbon dioxide under pressures ranging from 0.5 MPa to 5 MPa. It was found that factors such as coal rank and moisture content affected coal sorption behaviour as well as coal matrix changes. Harpalani (1989) conducted a series of sorption tests and found a relationship between gas sorption and changes in permeability. He confirmed that by desorption of coal gas, the coal matrix shrinks and the permeability increases. Milewska-Duda, Cegarska-Stefanska and Duda (1994) studied the swelling of the coal matrix due to methane sorption at pressures from 0.5 MPa up to 4.5 MPa and at 298 K temperature. They claimed that coal matrix expansion caused by gas sorption plays an important role in determining mining method so far as outburst phenomena are concerned. Adjoining figure indicates that at lower pressures the empirical measurements were in agreement with the theoretical data, but deviations were observed at the higher pressures. The compression of cleats and pores was recognized as the reason for this difference. As can be seen from Figure, at the lower pressures the amount of expansion was dependent on the direction and expansion of the cleats. The expansion perpendicular to the bedding was more than in the parallel direction. At higher pressures the difference decreased again as a result of the cleats and pores closing. Seidle and Huitt (1995) measured the shrinkage of the coal matrix with respect to desorption and the changing permeability of coal. They postulated that coal matrix shrinkage was more correlated to gas content than to gas pressure. They found that the amount of volumetric change depends on coal rank and sorbed gas type. It should be mentioned that their samples were placed in an oven at 48oC, which is significantly higher than the temperature of 20oC that is commonly used for tests. However they did not clarify the reasons for the more rapid desorption than adsorption. In adsorption it takes a longer time for the gas molecules to get adsorbed in macropores and reach the end of their path in the pore space and micropores, however, in desorption the molecules that are in the vicinity of the free surface and in macropores are easily desorbed and leave their places immediately. Then the inner molecules will be able to quickly flow toward the surface until equilibrium is established. Levine (1996) suggested that with gas sorption during the gas drainage process, the coal matrix shrinks and causes permeability to significantly increase. Different factors affecting this phenomenon include coal rank, petrographic composition, mineral matter content and composition of gases. Different coals exhibit different shrinking behaviour and it is crucial to note that one of his significant results was that carbon dioxide causes a greater degree of coal matrix change compared to methane. This is one of the basic principles of carbon sequestration projects. Exposing coal to CO2 causes different amounts of strain compared to methane or helium, and this difference is attributable to the different sorption capacities of the particular gases. Levine (1996) also studied the mechanical strength of coal, which was described as the ability to resist stress and change in dimensions. The figure opposite is the schematic representation of effective mechanical forces model of a coal bed reservoir. Harpalani and Chen (1997) studied the drainage of coal gas and its impact on the coal matrix and permeability. Based on their test results, they presented a relationship between changes in permeability and volumetric strains in the coal matrix, which was described as:
Volumetric strains also have a proportional relationship to the desorbed coal gas. Harpalani and Chen (1992) introduced the following equation:
In the conclusion to the results of their research they stated that: first, the permeability of coal was drastically increased by decreasing the gas pressure; second, by decreasing the fluid (gas) pressure the effective stress increases and tends to reduces the permeability, but coal matrix shrinkage influences the permeability and limits the decrease; third, permeability changes due to coal matrix shrinkage are dependent on the volumetric strain Results of the research of Ceglarska-Stefanska and Holda (1994) on sorption of various gases by coal matrix are shown It is obvious that the volumetric changes for coal matrix due to Helium gas were negligible, followed by Hydrogen gas which caused 0.05% swelling at 10 MPa, with nitrogen and Argon causing swelling amounts from 0.15% to 0.18%, respectively. The highest amount of swelling was related to Methane, which resulted in about 0.36% swelling. They concluded that by increasing the molecular weight of the gas the percentage of volumetric change would increase. However, one of the parameters, which was not mentioned, is the different affinity of those gases to the coal matrix, this is an important factor which should be taken into account. Figure opposite indicates that there isn’t any interaction between Helium and the coal matrix, however, coal adsorbs methane immensely. f) Studies in 2000 – present:St. George and Barakat (2001) in their experiment using sub-bituminous coal demonstrated how desorption of coal gas affected the coal matrix and effective stress. The experimental set up was similar to the Harpalani and Chen (1995) apparatus. Tests were conducted on cylindrical, 54 mm diameter core coal samples and in four different gas environments. Gases used included CO2, CH4, N2, and He. They found coal swelling due to sorption of carbon dioxide was about 12 times greater than for nitrogen and 8 times more than for methane. They also mentioned that the swelling due helium was negligible. They postulated that the strength characteristics of coal could be affected by compressive strains due to gas pressure reduction and coal matrix shrinkage. Also, in the presence of carbon dioxide, the coal underwent an initial contraction for a period of less than 45 seconds due to the hydrostatic pressure, which was then followed by expansion due to gas sorption. However, based on the experimental studies, this initial shrinkage time period was found to be shorter than that reported by St. George and Barakat (2001). |A later study, Chikatamarla, Xiaojun and Bustin (2004) examined the shrinkage and swelling of various Canadian coal samples with different ranks from sub-bituminous to medium volatile coals. The tests were made with regard to determining the capacity of the various coals for sequestration by adsorption. By using various gases, CO2, CH4, H2S, and N2, they demonstrated that the volumetric strains are proportionally related to the amount of adsorbed gas. H2S caused larger volumetric changes than the others, up to 15 times greater than carbon dioxide, 20 times more than methane and about 40 to 130 times more than nitrogen. In the second part of their experiments they compared the swelling and shrinkage of the coal matrix by introducing various gases to determine the effect on coal permeability. They reported that, by injecting carbon dioxide into the coal seam, the relative swelling of coal was markedly greater than the shrinkage of the coal matrix.
|
Fracturing is the basic element of tectonic disturbance. The process of folding gives rise to fracturing in beds. Cleats are natural fractures in coal. Methane and other coal seam gases will flow out of pores of coal if there is a pressure gradient acting as a driving force and the fractures are sufficiently open or permeable. When the pressure in the coal seam is reduced through mining, gas begins to desorb and migrate through the coal matrix and through natural fractures such as cleat. Natural fractures in coal can be divided into two main classes.
-
Micro and Macro cleat systems and Joints
-
Large Joints and shears
Cleats together with the bedding planes result in anisotropic behaviour of coal, both from the geotechnical and gas filtration points of view.
There are two mechanisms for the origin of cleat formation in coal.
- Endogenetic cleat: This is formed during the process of physical changes in the properties of coal during the metamorphic process. Coal matter undergoes density changes and a decrease in its volume. These processes are associated with the changes in the internal stress system, compaction and desiccation, and the formation of cleat planes.
- Exogenetic cleat: This is formed as a result of the external stresses acting on the coal seam. These include tectonic stresses, fluid pressure changes, folding and development of tensile stresses to which the coal seam is subjected during various time periods.
Endogenetic cleats are normal to the bedding plane of coal and generally occur in pairs. There are at least two sets of near perpendicular fractures that intersect the coal to form an interconnected network throughout a coal-bed. These two fracture systems are known as face and butt cleats. The shorter butt cleat normally terminates at a face cleat, which is the prominent type of cleat as can be seen in the adjoining figure .
The angle between the face and butt cleat is around 90 degrees.The spacing between cleats varies according to factors such as the coal maturity, the mineral matter and the carbon content, but normally is less than 25 mm but possibly more in dimension. The approximate width of the aperture and the length of the face and butt cleat spacing in some Australian coal samples are given in the table below.
|
Cleat |
spacing |
|
Face cleat spacing |
10-25mm |
|
Butt cleat spacing |
10-22mm |
|
Aperture |
0.1 – 2mm |
Coals with bright lithotype layers, with a high percentage of vitrinite macerals, have greater amount of cleats than dull coals. Common understanding is that cleats are formed due to the effects of the intrinsic tensile force, fluid pressure, and tectonic stress. The intrinsic tensile force arises from matrix shrinkage of coal, and the fluid pressure arises from hydrocarbons and other fluids within the coal. These two factors are considered to be the reasons for endogenetic cleat formation. On the other hand, the tectonic stress is regarded as extrinsic to cleat formation and is the major factor that controls the geometric pattern of cleats. Face cleats extend in the direction of maximum in situ stress, and butt cleats extend in the direction of minimum in situ stress which existed at the time of their formation. This is why regular cleats are formed in face and butt pairs. In general three sets of cleats are present in coal: face, butt and sometimes curviplanar cleat direction, which intersect both face and butt cleat as shown in the Figure.
In normal coal, gas desorbs from the internal coal surfaces to diffuse through the coal matrix and micropores to cleats and then enters the laminar flow regime. Cataclastic coals, which result from the formation of sheared coals through a brittle deformation mechanism, have interconnected pores and continuous cleats. They are divided into blocks of sizes intermediate between the cleat and microfractures size. It is clear that, in as much as the dimensions of these blocks are smaller, the mean diffusion distances are shorter, implying that the gas quickly reaches fractures and cleats for laminar flow. There are three different stages in the transport of gases through sheared coals. The first stage involves diffusion from and through the micropores to microfractures. Secondly, the flow of gas proceeds through microfractures to cleats or fractures, and the last stage is gas movement through cleats and fractures to the open surface. Ductile deformed coals are called mylonitic coal and are located in special structural positions such as thrust ramps and small-scale folds. In this kind of coal all fractures are tightly compressed, which means less ability to conduct the gas as well as a vast specific surface area, which is the specification of a good gas reservoir. This type of coal always exhibits low connectivity and results in the appearance of high pressure gas pockets, which represent one of the most well defined outburst prone places in coalmines.
Larger fractures are called joints and are found to extend over the whole or part of the coal seam and are much less frequent than cleat. These are of the exogenic origin and are related to tectonic movement. The frequency of joints increases rapidly when approaching shear structures and faults, large joints running over several times the thickness of the seam and at low angle to the coal are of endogenetic origin. Joints can cut across the lithological boundaries in the seam, but are in general limited to the seam thickness. Increase in their frequency is an indication of an approaching geological structure.